Glycan-Based Cell-Cell Communication
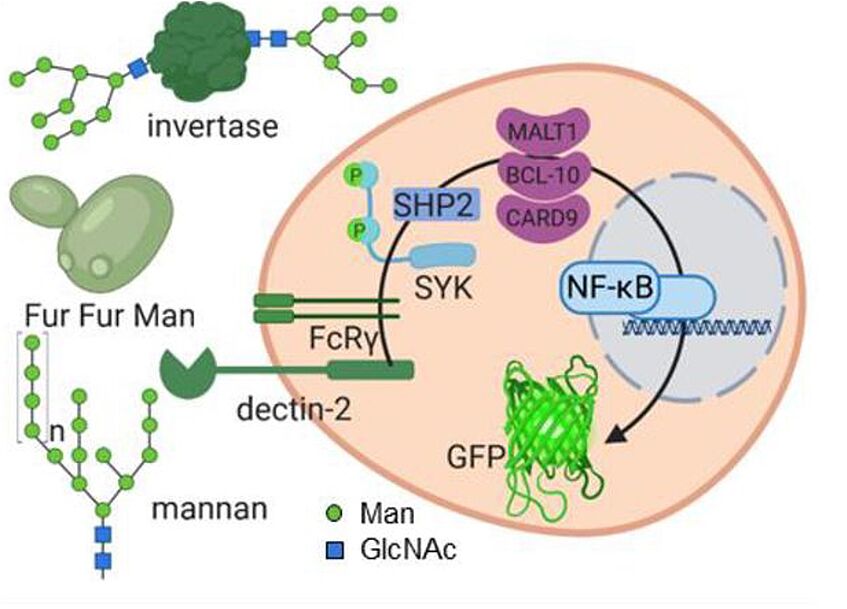
Reporter cell system for observation of glycan-lectin interaction (Fuchsberger et al., 2021)

This research is funded by the ERC (GLYCONOISE).
Every living cell is decorated with a dense fur of glycans. Multicellular organisms make use of this matrix to encode for specific information such as cellular identity, metabolic and activation status, and circadian clock. Cell surface glycans, present on glycoproteins and glycolipids modulate protein localization, their interaction partners and receptor life cycles. Since glycans are secondary gene products, their formation as well as their breakdown is determined by many factors, not limited to gene expression of potentially over 200 enzymes and transporters. Regulatory circuit arose on many different time scales: very quick alteration of the glycocalyx can be introduced using secreted hydrolases and endo- and exocytosis of glycoproteins. Long-term remodeling of glycans can result from gene expression and histone modifications (further reading Moremen et al.). How can such a specially and temporally regulated, complex and stochastic system encode reliably for information that can faithfully be decoded by other cells through the use of lectin receptors? To address this intriguing question, we have developed glycomimetic tools, small molecules that specifically bind to selected mammalian lectins. These tool compounds are used in combination with nano- and microparticles to ask very fundamental questions such as: How often does a protein-carbohydrate interaction on a cell surface lead to a productive, biological response? How do lectins and glycans find each other on two-dimensional surfaces? How much information can be transferred through such a communication channel and how does this compare to glycan-independent pathways of cell-cell communication? In the framework of the ERC-funded project GLYCONOISE we are investigating how information can be encoded into glycan structures and how this data can be decoded by cell surface exposed lectins.
Further reading
Fuchsberger, F. F.; Kim D.; Baranova, N.; Urban H.; Kagelmacher, M.; Wawrzinek, R. Rademacher, C.
Information transfer in mammalian glycan-based communication. eLife 2021, 12, e69415
